Pharmaceutical Packaging Material Technology – Glass
Pharmaceutical Glass Packaging
Glass is the name given to all amorphous bodies that are obtained by lowering the temperature of a melt independently of its chemical composition and the temperature range of solidification, which as a result of the gradual increase of viscosity adopts the mechanical properties of a solid body. For a large number of pharmaceuticals, including medicinal products for oral and local administration, glass containers are usually a safe choice. Different type of glass may be necessary depending on the characteristics and the intended use of medicinal products concerned.
Composition of glass and type
Glass can be formed bymixing together various inorganic substances. Usually these substances give ahomogeneous molten mass upon heating that can make in to different structures. Normally sand, soda ash and limestone is mixed in to 15; 5;4 ratio and heat up to 1500 Celsius to obtainnormal alkali glass. Thecolorof the glass may vary according to the adding additional substances.
Table 01 : Substances include in different colored glasses
| Color | Additional substance |
| 1. Colorless 2. Amber Color 3. Yellow 4. Blue 5. Green 6.Opal | Decoloriser such as selenium or cobalt oxide Carbon and sulphur or iron and manganese oxide Cadmium and sulphur Cobalt oxide or occasionally cupric oxide Iron oxide, manganese dioxide and chromium dioxide Fluorides or phosphates |
There are 4 main types of containers
1. Type 1
2. Type2
3. Type3
4.mp-soda glass (non- parenteral use) or European type4
Table 02: Main constitution in different type of glasses
| Type | Main constituents | Uses |
| 1.Type 1 Borosilicate glass E.g.- Pyrex Brosil | Sio2 66-72% Al2O3 4-10% Na2O /K2O 7-10 % B2O3 9-11% CaO 1-5% BaO 0-3% | 1.For lab glass apparatus 2. for water for injections |
| 2. Type 2 (treated soda lime glass) | The surface istreated with acidic gasses like SO2 at elevated temperature (5000 Celsius )and moisture | 1.for alkali sensitive products 2.foe infusion fluids ,blood and plasma 3. for large volume containers |
| 3. Type 3 (regular soda lime glass ) | SiO2 Na2O CaO | 1.for all solid dosage forms as tablets and powders |
| 4.Type mp | For all topical containers |
Properties of glass
1.High degree of chemical inertness
There is high chance that HCL attack to glass
There are some factors whichinfluence the degree of chemical attack on glass such as
-chemical composition of the glass
-temperature of attacking agent
-time in contact
This chemical inertness is more important incase of insulin.so treated neutral glass is also available.
2. Completely impermeable to all gasses, solutions and solvents.
Amber color glasses are resistant to UV and absorb IR
3. Glasses are lighter than metals
As an example soda glass has a density of 2.5 and borosilicate glass 2.25
But the rigidity and ability to withstand top weight is particularly good.
4. Good against thermal shock
Borosilicate glass is the common example
5. Glass surface is smooth
This makes cleaning easy and generally restricts surface damaging or scratching.
6. Glass is strong in compression but weaker in tension
7. If require for reuse can easily relearned
Recycling also can be satisfactorily use
8. Glass can be easily shattered, fractured and broken bysharp impacts and broken glass creates severehazardous for people or animals
9. Do not impact taste or odor to the product
10. Present low microbial risk
Manufacturing of Glass Containers
Four basic processes are used in production of glass containers.
1)Blowing – It uses compressed air to form the molten glass in the cavity of a metal mold
2)Drawing – Molten glass is pulled through dies or rollers and it produce Rods, Tubes, Sheet glass and other items of uniform in diameter. Ampoules, Cartridges and Vials are drawn from tubing.
3)Pressing – It uses the mechanical force to press the molten glass against the side of a mold
4)Casting – It uses the gravity or centrifugal force to initiate the formation of molten glass in the cavity
Glass containers produce in industry using mainly three methods.
- Blown glassware
- Tubular glassware
- Pressed glassware
Blown glass
This method classified into two main processes.
- Blow and Blow method
- Press and Blow method
Blow and Blow process
In this process the gob is dropped into an open blank or parisonmould. The neck is formed by top blow and then the parison or blank is blown from the base. The blank shape supported by the transferring is then transferred to the finishing mould where it is blown into the final shape. In this process IS (Individual Section) machinery is employed. It may have 4, 5, 6,8,10 or 12 stations and gobbing may be double triple or quadruple. The last can give speeds of over 400 containers per minute.
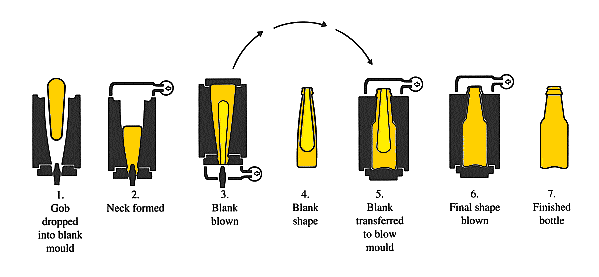
Press and Blow process
It may be used for wide mouthed (narrow necked) containers. The first stage is a gob feed into a mould which is followed by a plunger descending or ascending to form both the neck finish (at end) and parison shape. The blank is then transferred to a finishing mould where it is blown into the final shape.
These process can be automatic,semi –Automatic or handmade.Then all glass containers pass through an annealing lehr prior to final inspection. As glassware emerges from the lehr hand or automatic sorting is required. To carryout automatic
sorting operations the containers are marshaled onto a single line conveyor, for electronic or mechanical checking for body dimensions, bore, and visual damage (using imaging techniques). In hand sorting each container should inspect for damaging and the lead to packaging. The sorting area should maintain under positive air pressure in order to assist cleanliness.
Tubular glassware
Glass tubers are made by the Danner process or the Vello process.
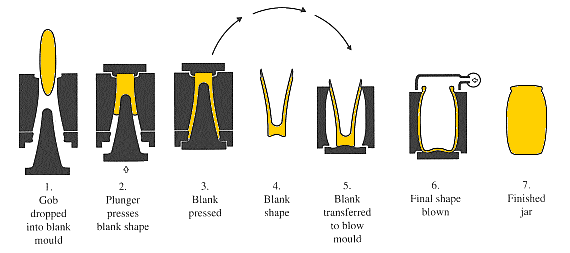
In the Danner process glass flows as a ribbon from a forehearth.Then it is falls on to the upper end of an inclined refractory sleeve carried on a rotating hollow shaft or blow pipe. The ribbon is wrapped around the sleeve to form a smooth glass. Then it is flows down the sleeve and over the tip of the shaft. Tubes are formed by blowing air through a blowpipe and rods are made by using a solid tip on the shaft. Then the tubes are drawn over a line of support rollers by a drawing machine.
InVello process glass is flowed from a furnace forehearth to a bowl which a hollow vertical mandrel is mounted or a bell surrounded by an orifice ring. The glass flows in a conical path between the bell and the ring and then travel through a set of rollers to a drawing machine up to 120m away. Tubing is made by blowing air through a bell which contains hollow tip and rod. A rod is produced by using a bell with a solid tip.
During the process tubing is gauged, classified for use and cut in to lengths.
Containers are made by cutting the tube in to pieces. Then flaming and shaping of each end is done. Then they are annealed. Tubular glass containers have a very thin wall and so has a lighter weight than blown glass. Ampoules, vials, cartridge tubes and prefilled syringes can be manufactured using this method.
Tubular glass containers are made in neutral type 1, surface treated type 1 glass, soda glass and etc. They are syliconized as a separate step after manufacture.
Pressured glassware
Pressured glassware is rarely used for packaging containers. When manufacturing pressed glassware the glass is flow through a plunger from a forehearth.In here the molten glass is mechanically pressed in to a plain or engraved mold by using a plunger.
Special pharmaceutical containers
Bottles and jars are used for drug and cosmetic product packaging. Many of the first specialized containers bore names which become associated with the pharmaceutical industry. Glass containers divided into narrow necked and wide necked container.
Tubular Glass Containers
Charles Danner introduced the first method of continuously drawing glass tube and it lead to the greater use of containers made from tubular glass Because it has lower weight , thinner and more even wall control , competitiveness , an ampoule met the need for sterile unit dose products and also it has ability to produce a hermetically sealed pack , ampoule
Ampoules
Ampoules can be broken at the neck restriction either by scoring or by having a ceramic point, baked on during the manufacture thus causing a weak point .Ampoules having colored break point led to an alternative where the ampoule is scored and then has a colored ring above or below the score to indicate the break point. This would help to avoiding colored contamination. Open paint cut ampoules are often now preferred.
Presealed ampoules can be used without a washing process. It needs special equipment to handle these, so that the vacuum retained in the sealed ampoule doesn’t draw in glass particles when it is opened. Flamed opening method can neutralize the vacuum it called as preheating. Washing is otherwise an essential stage in the use of ampoules and specialized machinery with various combinations of ultrasonic vibration and jets of water, steam or air is available for this purpose
Ampoules sterilized by dry heat, steam autoclaved after filling provided the product will withstand this process sealing and filling ampoules are inspected for seal and visual contamination. The seal or leakage test was performed in dye solution under vacuum .High voltage tests which detect through cracks. Presents of particulate matter or glass spicules can be viewed by either direct light or polarized light but this is not a ideal for use in regular quality control test.
Ampoules are produce as preprinted or printed after filling. In preprinted stage ceramic ink are printed by the screen process and then fired on. Printing after filling gives greater stock flexibility but requires extra security precautions during the time when the ampoules bear no identification. It was one of the first unit dose containers and anticipated that alternatively prefilled syringes will ultimately take over a major share of the ampoules market fusion of glass ampoules tested by immerging in a solutioncontaining a dye and applying a vacuum. But now this can be superseded by electrical conductivity testing or capacitance testing.
Vials
Parallel side container with a flat or concave base with a variety of neck finishes. The advent of the multidose injection vial with its rubber plug and aluminiumovercap increased in use.Intravenous solution, multidose vial product rely use of rubber closure because it became over cap . The metal screws cap or seal in use later because it is clinched under a bead.
The closure must make an air tight seal and maintain sterility and compatible with the product, not extracting or absorbing from the product or imparting anything to it. It must reseal after needle is removed. The range of rubber material includes natural and synthetic base materials.
Cartridge tubes, Disposable syringes
The use of a glass tube with an end cap seal and movable plunger is another early use of a unit dose injectable. When plastic disposable syringes become available, glass cartridge tubes became the first obvious choice, thus leading to high volume quantities .The next stage combine cartridge tube and syringe creating a glass disposable syringe.
Syringes contain 2 parts of a pharmaceutical formulation to be kept separate and then mixed immediately prior to use with pack relying on a rubber piston to effect the injection , this must both release without sticking and then more smoothly with the minimum of force . This may be assisted by lubricants in the rubber which subsequently migrate to give a surface film by siliconising or coatings.
Aerosols
Use of glass bottles for aerosols has received mixed comments on the risks involved .Although glass offer greater flexibility in design than cylindrical metal cans, It is essential that the breakage risk is safe guarded against either by good bottle strength .uncoated glass bottles are usually used in conjunction with low pressure aerosols .Glass aerosols inevitably cost more than metal cans but offer plus features on appearance which is highly desirable for toiletries and cosmetics.
Test for glass container
Mainly there are two tests for glass container. Those are surface hydrolytic resistance and hydrolytic resistance of powdered glass.
Surface hydrolytic resistance test is conducted on unused glass containers.Initially container is rinsed three times with carbon dioxide with free water .Then the container is allowed to drain and it is filled with the carbon dioxide free water to the required volume. If ampoules are used, they are sealed by heat fusion. The container temperature is maintained between 100oC to 120o C over 20minutes. Finally the temperature is lowered from 120oC for 40 minutes.
Hydrolytic resistance of powdered gas test is from the glass containers, alkalineconstituents are leached into purified water under the conditions of elevated temperatures. When the glass is powdered the leaching of alkali can be enhanced in the powdered is critical. The principle involved in the powdered glass test in estimate the amount of alkali leached form the glass powder .The basic analysis is acid –base titration using methyl red indicator.
Table 03 : Advantages and disadvantages of glass containers
| Advantages | Disadvantages |
| 1.Glass is 100% recyclable and that means can be holding a glass bottle that is over 100 years old 2.It is transparent ,can be see what is inside 3.Water and gas barriers 4.Inertness to chemical substance 5.Long term resistance reputation as high grade material 6.Effective closure and resolves are used | 1.Difficult in transportation 2.Fragile and costly, cannot be fixed if it is broken or damaged 3.Loss of nutrients due to effect of light 4.Washing process necessary 5.Glass cannot remain in wet condition when it is stacked together because it will cause deterioration |
References
Dean DA,EvansER,Hall IH;(2000);Pharmaceutical Packaging Technology; first edition; Taylor &francis; New york;page 147-185
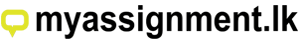

Leave a Reply